TRACK 2: From Bench to Bedside: Bioanalysis and Translational Science
Category: Poster Abstract
(T1430-02-07) Advancements in Free Heme Analysis: Utilizing a Co-Solvent to Simplify Sample Preparation
Tuesday, May 6, 2025
2:30 PM - 3:30 PM ET

Matthew Nolan
Research Associate
Prolytix, United States
Joey Campbell, Ph.D.
Senior Scientist
Prolytix
Essex Junction, Vermont, United States
Presenting Author(s)
Main Author(s)
Purpose: Researchers have been interested in identifying and quantifying free heme in physiological matrices for decades. The reason for this is the toxicity of heme and the ability to act as a hemolytic and proinflammatory molecule when not bound to hemoproteins. Unfortunately, differentiating between bound and free heme is challenging as many sample preparations strip away heme from these vital hemoproteins causing inaccurate measurements of the free heme in plasma samples. The introduction of a co-solvent has shown to be useful to keep free heme soluble while precipitating the bound heme with their respective hemoproteins. Free heme in the supernatant is injected onto an Ultra Performance Liquid Chromatography (UPLC) system and separated using a high pressure reversed phase column and quantified using the peak area of chromatograms.
Methods: All chromatography runs were executed on a Waters UPLC system equipped with a UV detector set to 398 nm absorbance. Following sample preparation, samples were injected and the respective heme signal from the chromatogram was evaluated. The free heme concentration in the sample is extrapolated from a calibration curve composed of commercially available heme. Unlike the pH modified acetone precipitations proposed in literature, this protocol uses a co-solvent DMSO-Methanol mixture. While heme has limited solubility in many matrices, it remains completely soluble in DMSO. Plasma proteins are known to precipitate out of solution in cold methanol. Free heme is partially soluble in methanol and completely soluble in DMSO. Using the co-solvent allows for free heme to solubilize in DMSO while the respective heme-bound hemoproteins precipitate out from the methanol portion of the mixture. Precision, accuracy and specificity experiments were crucial for evaluating this phenomenon. Specificity was evaluated through a comparison of respective peaks and relative retention times of heme in diluted plasma compared to a commercial source of heme. These experiments consisted of diluted plasma spiked with excess heme solution to be run through a molecular weight cut off filter, precipitated using the co-solvent mixture and diluted 4-fold into mobile phase. The results of this informed subsequent spike and recovery experiments to evaluate precision and accuracy. Refer to Table 1. Precision and accuracy were evaluated based on the percent relative error (accuracy) and the percent coefficient of variance (precision) of the measured free heme compared to the spiked quantity of the sample. Samples were evaluated with spiking before the precipitation step and after the precipitation step to verify quantification and removal of heme that was bound to the hemoproteins. Plasma samples were spiked with 5 different levels of free heme from a commercial source and analyzed on the UPLC.
Results: Specificity results demonstrated the limited solubility of heme as well as the added benefit of the co-solvent to precipitate out heme bound complexes. Heme spiked plasma samples showed no comparable signal when run through the MWCO filter as the spiked heme precipitated out in the mobile phase. Additionally, there is a prominent signal for the precipitated sample and a saturated signal with the sample that was diluted with mobile phase. Refer to Figure 1. Precision and accuracy results were compared to typical acceptance criteria and passing with average coefficient of variation ≤ 20% across all runs and overall relative error was within 10%. Refer to Table 1 and Figure 2. The spike and recovery assessed before and after precipitation supports that the free heme spikes were bound by hemoproteins in the plasma sample and subsequently precipitated in the bound form using the co-solvent.
Conclusion: We have developed a suitable method to measure free heme in plasma samples. The use of a co-solvent as a precipitation solution simplified the sample preparation and shows promise in differentiation between free and bound heme in plasma samples. The alteration of sample preparation method helps move free heme analysis forward to more precise and accurate analysis methods.
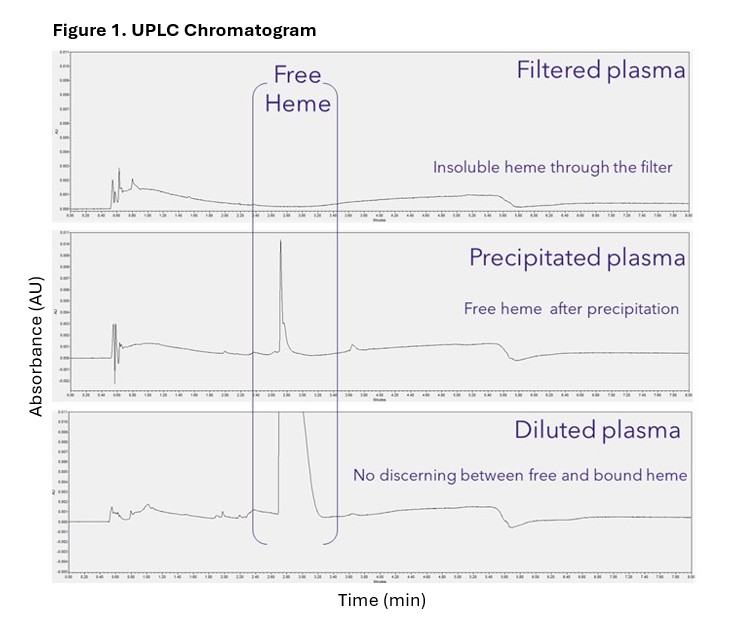 Figure 1
Figure 1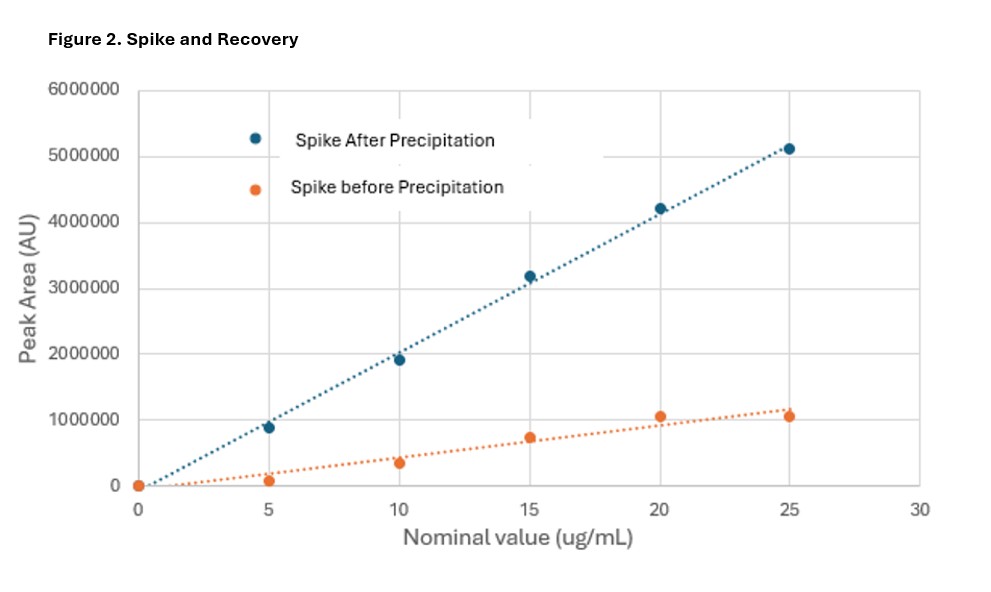 Figure 2
Figure 2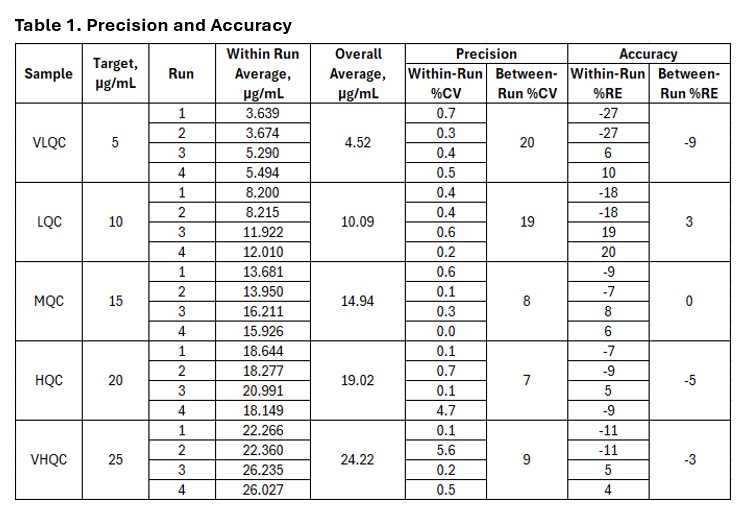 Table 1
Table 1